NIH-527 2008-2024 free printable template
Show details
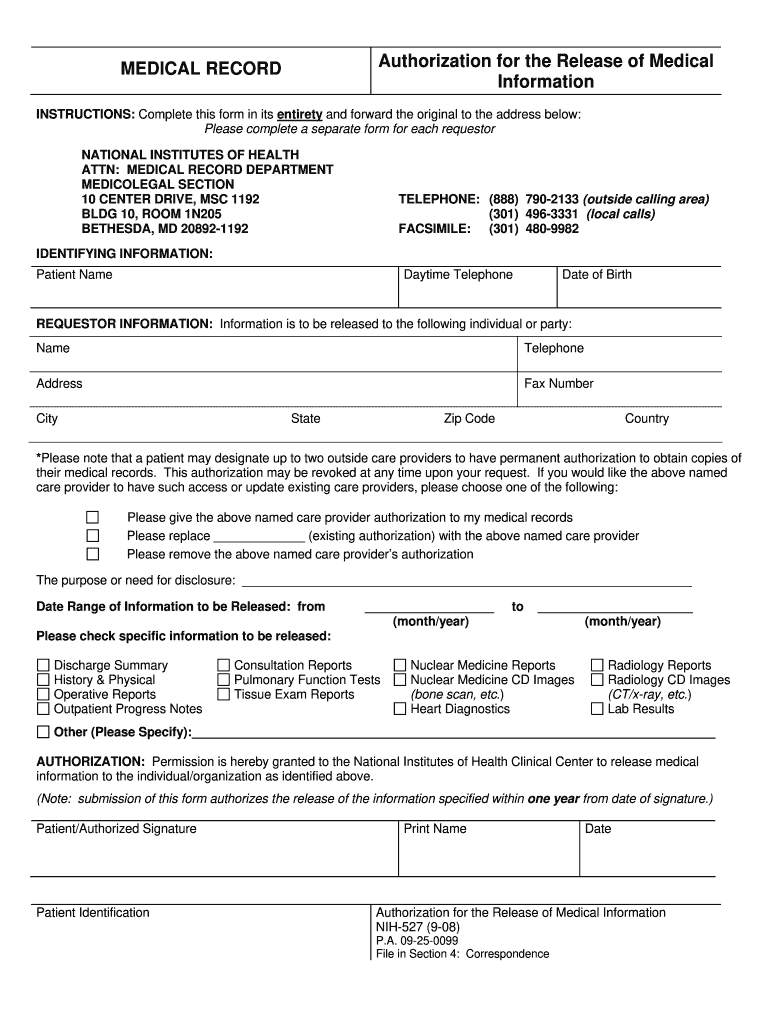
Patient/Authorized Signature Patient Identification Print Name Date NIH-527 9-08 P. A. 09-25-0099 File in Section 4 Correspondence. Authorization for the Release of Medical Information MEDICAL RECORD INSTRUCTIONS Complete this form in its entirety and forward the original to the address below Please complete a separate form for each requestor NATIONAL INSTITUTES OF HEALTH ATTN MEDICAL RECORD DEPARTMENT MEDICOLEGAL SECTION 10 CENTER DRIVE MSC 1192 BLDG 10 ROOM 1N205 BETHESDA MD 20892-1192...
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign

Edit your nih 527 form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your nih 527 form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit nih 527 online
Follow the steps down below to take advantage of the professional PDF editor:
1
Log in to your account. Start Free Trial and register a profile if you don't have one yet.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit nih 527 form. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, dealing with documents is always straightforward. Now is the time to try it!
How to fill out nih 527 form

How to fill out NIH records?
01
Start by accessing the NIH records system through their online portal or by obtaining the necessary forms from the NIH office.
02
Provide accurate and complete information, such as the project title, description, and objectives. Include any relevant background information or previous work on the project.
03
Fill in all required fields, such as key personnel involved, budget details, and funding sources. Make sure to review the instructions or guidelines provided to ensure all necessary information is included.
04
Include any supporting documentation or attachments required, such as CVs, letters of support, or consent forms, if applicable.
05
Carefully review all the information provided before submitting the records. Check for errors or missing information and make any necessary revisions.
06
Once completed, submit the NIH records either electronically through the online portal or by mailing the forms to the designated address.
Who needs NIH records?
01
Researchers and scientists who are applying for grants or funding from the National Institutes of Health.
02
Institutions or organizations that receive funding from NIH for research projects or programs.
03
Individuals or entities involved in collaborations or partnerships with NIH-funded projects may also need to maintain NIH records for documentation and accountability purposes.
Video instructions and help with filling out and completing nih 527
Instructions and Help about information authorization medical sample
Fill nih medical release form : Try Risk Free
People Also Ask about nih 527
Is NIH a reliable source?
Can anyone go to NIH?
What was the purpose of NIH?
How do I request records from NIH?
Who owns NIH?
What does NIH stand for?
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is nih records?
NIH Records is an online database of information related to research funded by the National Institutes of Health (NIH). It includes records on research projects, grants, scientific publications, and other information related to NIH-funded research.
Who is required to file nih records?
The NIH requires all NIH-funded investigators to submit final peer-reviewed manuscripts that have been accepted for publication in a journal to the NIH Manuscript Submission (NIHMS) system.
What information must be reported on nih records?
The information that must be reported on NIH records includes the grantee's name, project title, funding source, award amount, project period, project objectives/goals, progress reports, and any financial or technical reports.
When is the deadline to file nih records in 2023?
The exact deadline for filing NIH records in 2023 has not yet been determined. However, NIH typically requires that all records be submitted within 30 days of the expiration of the grant period.
How to fill out nih records?
To fill out NIH (National Institutes of Health) records, follow these steps:
1. Obtain the necessary forms: Visit the NIH website or contact the appropriate department to get the required forms for record submission. Make sure you have the right forms specific to the type of record you need to fill out.
2. Read the instructions: Before you start filling out the forms, carefully read the provided instructions. Understand the purpose of the record, the information requested, and any specific guidelines you need to follow.
3. Gather the required information: Collect all the necessary information you will need to fill out the NIH record. This may include personal details, project information, funding details, collaborations, research topics, etc. Ensure you have all the relevant documents and data handy.
4. Start filling out the form: Begin by entering your personal details such as name, contact information, and institutional affiliation. Pay attention to any fields marked as mandatory and ensure accurate spelling and formatting.
5. Provide project information: If the record you are filling out relates to a specific project, provide comprehensive details about the project. This may involve describing the objectives, methods, anticipated outcomes, and potential implications.
6. Include funding details: If the record requires information about funding sources, provide the necessary details regarding grants, sponsors, or other financial support received for the project. Include the names of funding agencies, grant numbers, amounts, and duration.
7. Add any relevant collaboration information: If you collaborated with other researchers or institutions on the project, provide their names, affiliations, and their contribution to the work. This is important to highlight collaboration and potential collaborations for future research.
8. Review and double-check: Once you have filled out the form, review it thoroughly for accuracy, precision, and completeness. Double-check all the information you have provided to avoid any errors or missing data.
9. Sign and submit: After verifying the accuracy of all information, sign the form, and submit it as per the provided instructions. Ensure you meet any associated deadlines or submission requirements.
10. Keep a copy: Before submitting the record, make a copy for your personal records. This will be useful for reference purposes and can help track your submissions.
Remember to consult with any specific instructions provided by NIH or the relevant department to ensure compliance with their requirements.
What is the purpose of nih records?
The purpose of NIH (National Institutes of Health) records is to document and maintain a comprehensive record of biomedical research and health-related activities conducted or funded by the NIH. These records serve multiple purposes, including:
1. Reference and Documentation: NIH records serve as a historical archive, providing documentation of the research projects, programs, and initiatives funded by the NIH. These records help researchers, scholars, and policymakers in the future to understand the context, progress, and outcomes of biomedical research.
2. Accountability and Transparency: NIH records help ensure accountability by maintaining an accurate record of the allocation of funds, grants, and contracts. These records provide transparency and help prevent fraud, mismanagement, or ethical violations in research.
3. Compliance and Regulation: The NIH is responsible for ensuring compliance with relevant regulations, policies, and guidelines. The records assist in monitoring and evaluating compliance with ethical standards, data management, human subject protections, animal welfare, and other regulations in biomedical research.
4. Reporting and Analysis: NIH records are used to generate reports, statistics, and analyses of research activities and outcomes. These reports help assess the impact, effectiveness, and progress of biomedical research funding, identify trends, and inform future planning and decision-making.
5. Intellectual Property and Patents: The NIH records play a crucial role in documenting and preserving intellectual property generated through NIH-funded research. They help researchers and institutions track and protect their inventions, discoveries, and patents, facilitating technology transfer and commercialization.
6. Research Oversight and Peer Review: NIH records are essential in supporting the peer review process for grant proposals and publications. They enable the evaluation of research proposals, the selection of reviewers, and the assessment of research progress, ensuring the highest scientific and ethical standards.
Overall, NIH records serve as a vital resource for the scientific community, policymakers, healthcare professionals, and the public by facilitating research, promoting accountability, ensuring compliance, and fostering transparency in the biomedical research domain.
What is the penalty for the late filing of nih records?
The National Institutes of Health (NIH) does not explicitly state penalties for the late filing of records. However, failing to comply with NIH regulations and requirements can have potential consequences. These consequences may vary depending on the specific situation and could include:
1. Loss of funding opportunities: NIH grants require compliance with reporting and record-keeping guidelines. Failure to meet these requirements may result in the loss of current and future funding opportunities.
2. Audit and investigation: Non-compliance with NIH regulations may trigger an audit or investigation. If irregularities or mismanagement of records are found, this can lead to further punitive actions.
3. Reputational damage: Late filing or non-compliance with NIH regulations can harm an institution or researcher's reputation within the scientific community, making it more challenging to secure future funding and collaborations.
While there is no specific penalty outlined by NIH for late filing, it is important to comply with all reporting and record-keeping requirements and adhere to established deadlines to avoid potential negative consequences.
How can I manage my nih 527 directly from Gmail?
nih 527 form and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How do I execute nih medical records request online?
Easy online nih medical records release form completion using pdfFiller. Also, it allows you to legally eSign your form and change original PDF material. Create a free account and manage documents online.
How do I fill out the nih form 527 form on my smartphone?
Use the pdfFiller mobile app to complete and sign nih medical records department form on your mobile device. Visit our web page (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, the capabilities you’ll have access to, and the steps to take to get up and running.
Fill out your nih 527 form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Nih Medical Records Request is not the form you're looking for?Search for another form here.
Keywords relevant to nih records form
Related to nih records request
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
























